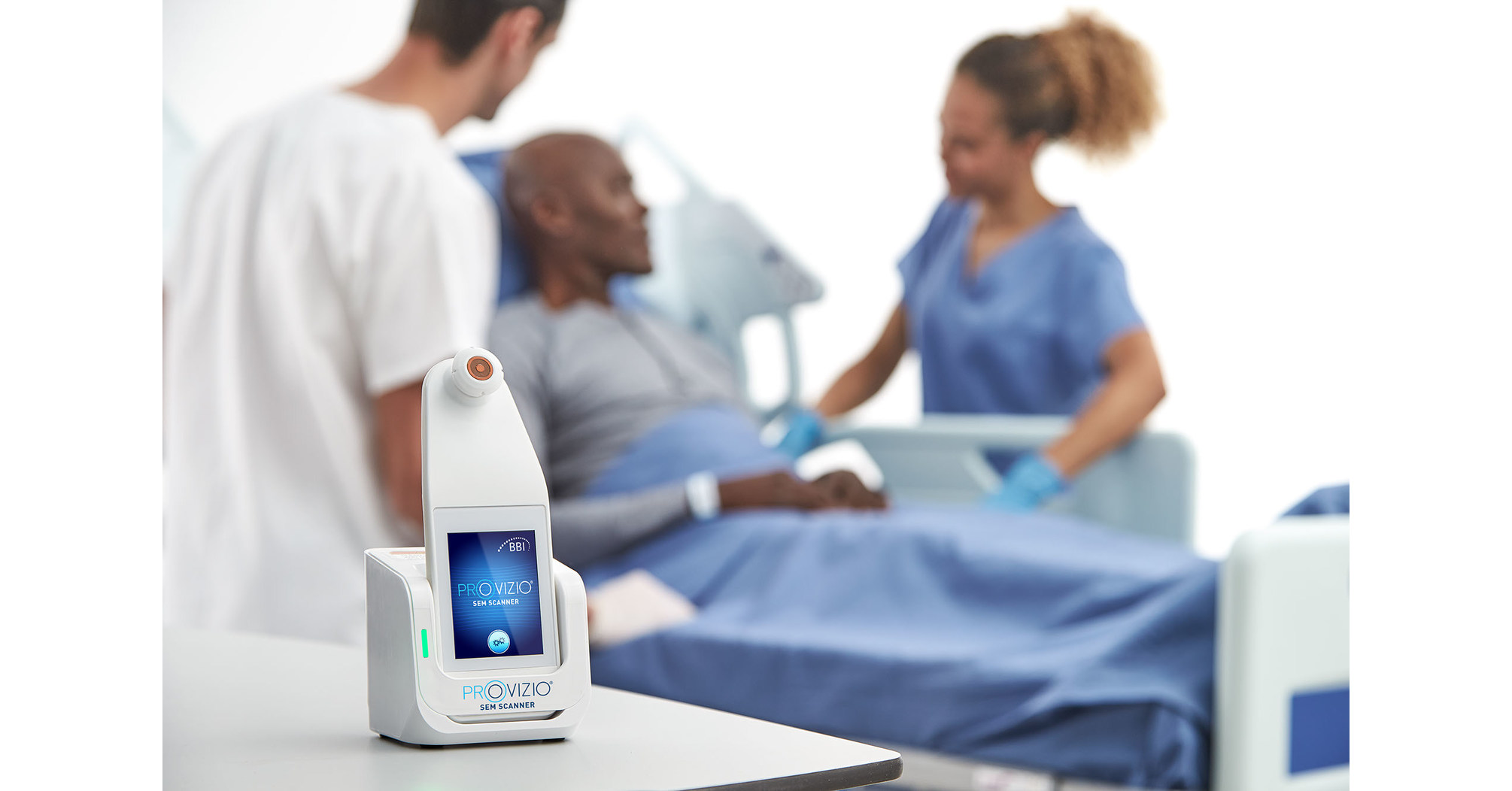
Bruin Biometrics, LLC (BBI), and Arjo (ARJO-B.ST) agree equity investment and exclusive worldwide distribution rights for pressure ulcer SEM Scanner
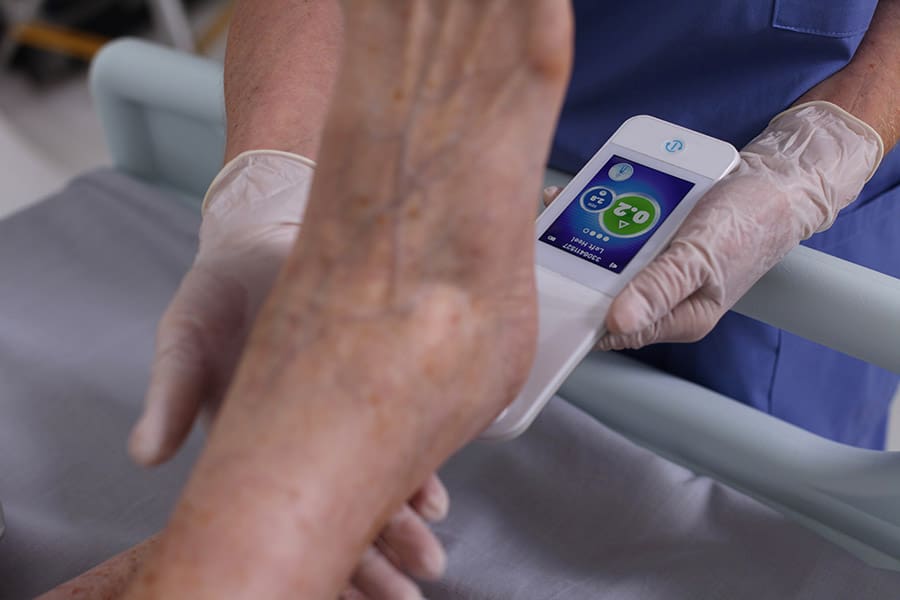
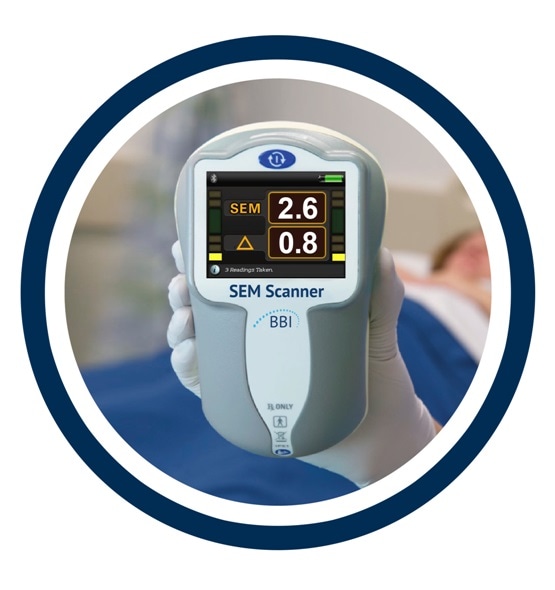
Clinical profile of the SEM Scanner — Modernizing pressure injury care pathways using Sub-Epidermal Moisture (SEM) scanning

Provizio® SEM Scanner Single Use Sensors Awarded Onto NHS Supply Chains Tower 5 Pressure Area Care framework - Bruin Biometrics

Early-Detection Technology Reportedly Helps Eliminate Pressure Ulcers, Per Patient Study - Rehab Management

Using Subepidermal Moisture Level as an Indicator of Early Pressure Damage to Local Skin and Tissue - Bruin Biometrics
FDA identifies this generic type of device as: Pressure ulcer management tool. A pressure ulcer management tool is a prescripti

Using Subepidermal Moisture Level as an Indicator of Early Pressure Damage to Local Skin and Tissue - Bruin Biometrics